Petroleum Chemistry
Introduction
Geologists use the science of petroleum chemistry to assist in exploration. From the composition of the oil and variations of properties in a trend or basin, they may find clues on how the crude was formed millions or hundreds of millions of years ago. Reservoir engineers are concerned with how the liquid and gas properties will change in the reservoir in response to fluid withdrawals. For the production and facility engineers, petroleum chemistry is vital in the design of wells, artificial lift and facilities. Corrosion and toxicity of the produced gas may be a critical factor in the design of facilities and wells. The environmental engineer, with a focus in safety, health and the environment, needs to understand the chemistry of the produced fluids and gases. This is particularly important today when new platforms are located in environmentally sensitive areas. Finally, the composition of the petroleum once it has been processed, will help determine its market price.
Hydrocarbons can exist in all three phases (gas, liquid and solid). Petroleum or crude oil is a naturally occurring, flammable, complex and variable mixture of hydrocarbons plus other organic compounds in the liquid state. Petroleum contains 83- 87% carbon, 10 to 14% hydrogen, with trace amounts of nitrogen, oxygen and sulfur. The sulfur content of oil can be as high as 6%. In general, crude oil with sulfur content in excess of 1% is considered sour crude. Under 0.05% content of sulfur, the crude oil is considered sweet, and less costly to refine, so it commands a higher price.
Petroleum Fractions
The PNA classes ( paraffinic, naphthenic and aromatics) of petroleum fractions are common classifications of the most common components in petroleum. Paraffins include the alkane series, naphthenes include the cycloalkanes and aromatics include the all compounds that contain one or more ring structures similar to benzene.
- Alkanes:
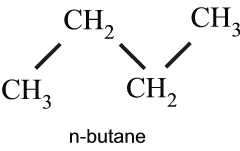
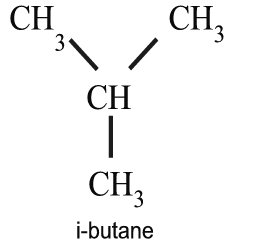
The series have single carbon bonds with the general formula, CnH2n+2. All alkanes after ethane have isomers, which have the same number of carbon and hydrogen atoms, with single bonded carbon atom. The n-butane is the normal single chain of carbons, while the i-butane has a Y arrangement, or a branched chain molecular structure. The isomers have identical molecular structure, but somewhat different properties.
 |
 |
Dry gas consists of mostly methane (CH4) and ethane (C2H6). Alkane components with higher molecular weights, C3H8 (butane) to C8H18 (octane), may be present in separator gas or wet gas at reservoir conditions. Properties of the alkanes are found in most reservoir engineering textbooks (see references at end). The table provide below, are alkane and impurities properties from Dake.
Component |
Molecular Weight |
Pc (psia) |
Tc (o R) |
State at T = 60 deg F and P = 14.7 psia |
|
| CH4 | Methane | 16.04 |
668 |
343 |
Gas |
| C2H6 | Ethane | 30.07 |
708 |
550 |
Gas |
| C3H8 | Propane | 44.10 |
616 |
666 |
Gas |
| i-C4H10 | i-butane | 58.12 |
529 |
735 |
Gas |
| n-C4H10 | n-butane | 58.12 |
551 |
765 |
Gas |
| i-C5H12 | i-pentane | 72.15 |
490 |
829 |
Liquid |
| n-C5H12 | n-pentane | 72.15 |
489 |
845 |
Liquid |
| i-C6H14 | n-hexane | 86.18 |
437 |
913 |
Liquid |
| n-C7H16 | n-heptane | 100.20 |
397 |
972 |
Liquid |
| C8H18 | n-octane | 114.23 |
361 |
1024 |
Liquid |
| C9H20 | n-nonane | 128.26 |
332 |
1070 |
Liquid |
| C10H22 | n-decane | 142.29 |
304 |
1112 |
Liquid |
| CO2 | Carbon dioxide | 44.01 |
1071 |
548 |
Gas |
| H2S | Hydrogen sulfide | 34.08 |
1306 |
672 |
Gas |
| N2 | Nitrogen | 28.01 |
493 |
227 |
Gas |
-Cycloalkanes
As the name suggests, these are similar to the alkanes, however there is one or more ring structures. The elements of the cycloalkanes are bound by single bonds.
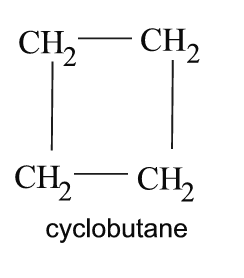 |
 |
- Aromatics
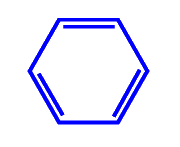
Often referred to as the benzene series, because the series has a similar cyclic structure to benzene. Benzene is C6 H6 the first compound of this series. The structure of benzene is shown in the two diagrams below. Both show six carbon atoms connected in a hexagonal geometry. One hydrogen atom is linked to each carbon atom The diagram on the left is more correct, as there is no double bond in benzene. The C-C bonds, were shown from X-ray diffraction to have all the same length. The electrons for the C-C bonds are equally distributed. Thus, to show the delocalization nature of bonding, the bonds are conceptually shown as a circle. This structure is common in organic chemistry.
The aromatics such as benzene, toluene, and xylene are important industrial chemicals, used in drugs, solvents, plastics, synthetic rubber and dyes.
Benzene is a well known carcinogen and as such, its use in gasoline is limited. Human exposure to benzene is a global problem. See Wikipedia link in the references.
Benzene with dislocated C-C bonds |
Benzene with alternative double bonds |
- Other non-hydrocarbon elements
Sulfur is found to some extent in all crudes and produced gas. It may be in the form of free sulfur, H2S and organic sulfur compounds. The lower API gravity crudes are more likely to contain sulfur.
Nearly all crude oils contain small quantities of nitrogen.
Helium can be present in trace amounts in natural gas.
Oxygen is also found in crude oils either as free oxygen or as part of a radical of a larger compound.
Kerogen
There is great interest in oil shale production. Kerogen or the kerabitumen is a mixture of organic chemical compounds that with heat can be converted to one or more hydrocarbon compounds. A chemical compositional description of kerogen is not possible. Generally, the organic compounds have very high molecular weights and are insoluble in normal organic solvents.
Enormous kerogen deposits have been found in shales. Typically, these have been referred to as "oil shale" deposits, which strictly speaking, kerogens are distinctly different from petroleum. Also, kerogen deposits are referred to as "green oil", in that the organic matter has not had sufficient time nor heat to transform it into petroleum.
Solids Precipitation
Hydrocarbons can exist as solids, liquids or gases. Solids can form in the reservoir, wellbore, pipelines and surface facilities. The deposited solids can consist of asphaltenes, waxes or a mixture of these components. Solids or highly viscous liquids may contain resins, crude oil, fines, sands and water.
- Asphaltenes
Resins and asphaltenes are subclasses of aromatics although some resins contain only naphthenic rings. Asphaltene precipitation during primary depletion occurs more commonly in light to medium oils, initially highly undersaturated. Maximum asphaltene precipitation occurs around the saturation pressure. Interestingly, heavier crudes with higher asphaltene content have less asphaltene problems because they can dissolve more asphaltenes. Asphaltene precipitation can also occur as a result of hydrocarbon or CO2 gas injection. The precipitation commonly occurs at the producer at breakthrough, however it can occur anywhere in the displaced fluids region.
The mechanisms of asphaltene precipitation and related models is given in the references at the end of this discussion.
- Wax Deposition
Waxes composed primarily of normal alkanes crystallize in large flat plates and are referred to as paraffinic waxes. Waxes composed primarily of cycloalkanes and i-alkanes form microcrystalline structures. Waxes form primarily to decrease in temperature and a weak relationship with pressure.
Wax precipitation does not necessarily mean deposition will occur. Wax crystals may disperse. Waxes may crystallize around fine particles such as asphaltenes or fines in the producing crude.
Methods to prevent or remedy wax deposits includes thermal, chemical and mechanical means. Commonly, scrapers are used to remove wax deposits in pipeline and wellbores.
References:
1. L.P. Dake, Fundamentals of Reservoir Engineering, 1978, page 16 for alkane and impurity properties.
2. Nghiem, Long, Asphaltenes and Waxes, Petroleum Engineering Handbook, Volume 1, pages I-397 to I-399.
3. Wikipedia - "Oil Shale"
4. Wikipedia - Petroleum